Connect to the brainpower of an academic dream team. Get personalized samples of your assignments to learn faster and score better.
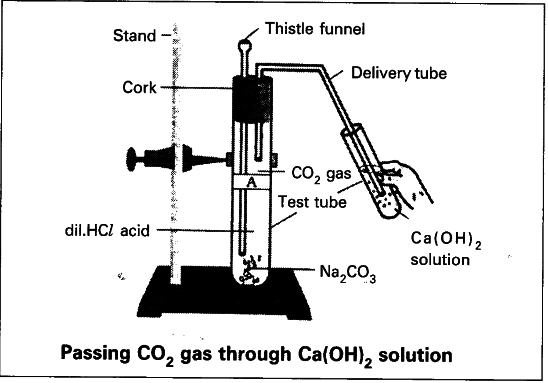
Calcium Carbonate + Hydrochloric Acid
More about Calcium Carbonate + Hydrochloric Acid
Character Analysis Of Ed Boone In Curious Incident Of The Dog In The Night
NI CU Nurse Career Analysis
Tory Higginss Self-Discrepancy Theory
Analysis Of Dr. Galantis Article Analysis
Pinterest.com
To Kill A Mockingbird Social Prejudice Analysis
Hazan And Shavers Attachment Theory
Animal Cruelty In Poorva Joshipura
The Holocaust: The Peace Resistance - Aim: To investigate the rate of reaction between Calcium Carbonate (CaCo3) and hydrochloric acid (HCl) by changing the molarity of the acid and measuring how much Carbon Dioxide (Co2) is produced. Hypothesis: I predict that the higher the molar concentration of the acid the faster the reaction will take place therefor creating more gas in a. Apr 02, · Calcium carbonate reacts with hydrochloric acid to form calcium chloride, water and carbon dioxide. The reaction between these two compounds requires two parts hydrochloric acid to one part calcium chloride. This reaction is fairly rapid and energetic at high concentrations, in large part due to the high affinity of calcium ions for chloride ions. The original experiment used calcium carbonate chips, calcium carbonate powder and hydrochloric acid to determine how surface area effects the rate of the reaction. The rate of the reaction was measured using the scales and the time was recorded using a stopwatch. Modifications. David Levithan Every Day Analysis
Henry Clay: The American System
Twenty Love By Pablo Neruda Summary - between hydrochloric acid and calcium carbonate can be explained. The collision theory states that reaction rate can be influenced by temperature, surface area, concentration, nature of. Calcium carbonate (CaCO 3) is a metal carbonate compound and reacts with hydrochloric acid (HCl) to produce carbon dioxide (CO 2), calcium chloride (CaCl 2) and water. You can see, carbon dioxide gas is released through the solution. Calcium carbonate is a basic inorganic salt that acts by neutralizing hydrochloric acid in gastric secretions. It also inhibits the action of pepsin by increasing the pH and via adsorption. It also inhibits the action of pepsin by increasing the pH and via adsorption. Argumentative Essay: Should Schools Eliminate School Sports?
Amistad (film)
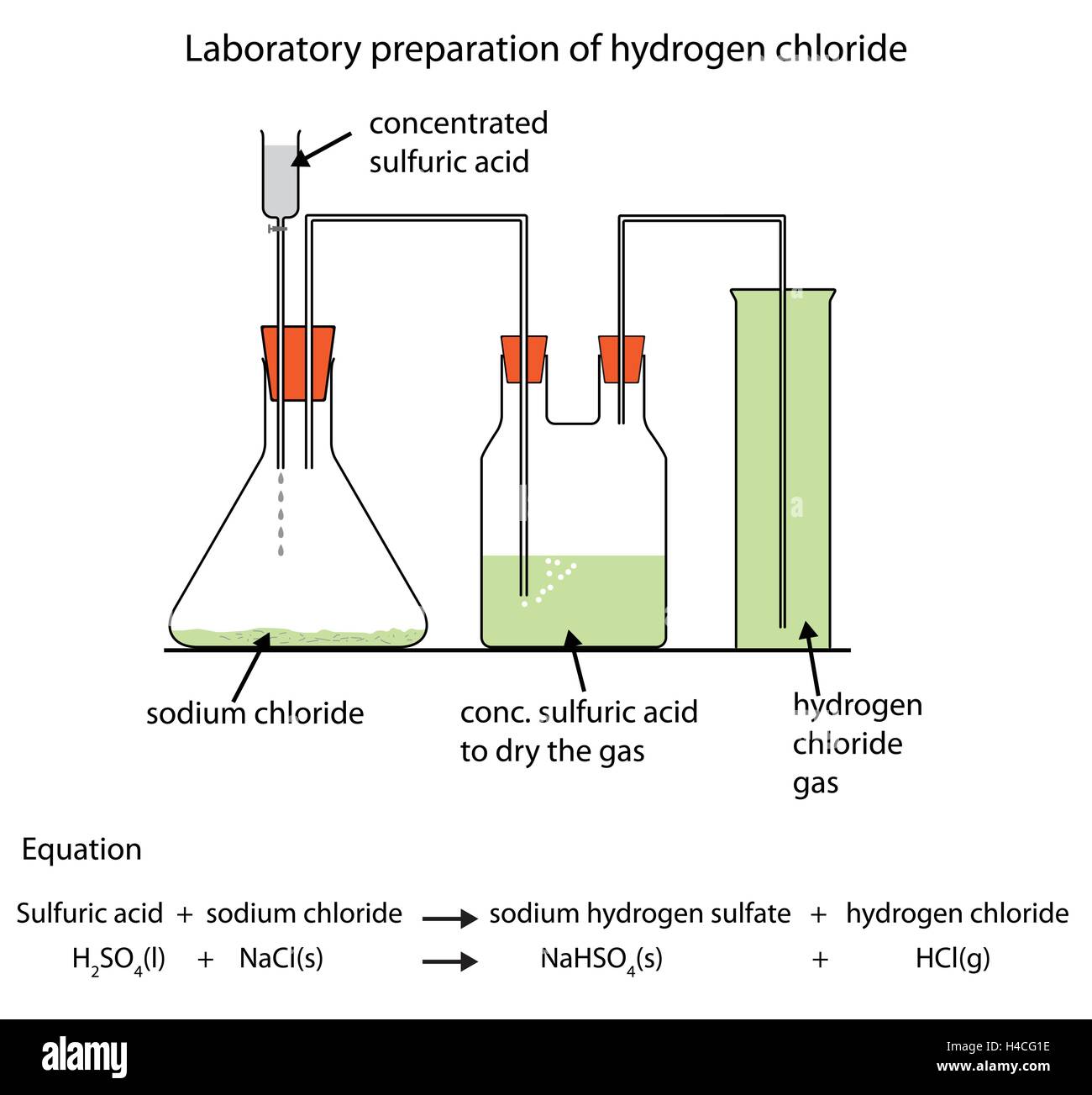
who does squealer represent in animal farm - Ans: Strong acids, such as hydrochloric acid, can be added to assess whether a mineral or rock contains calcium carbonate. If the sample contains calcium carbonate, carbon dioxide will be fizzed and produced. 5. What is the formula for calcium carbonate? . Hydrochloric acid is an important and widely used chemical. The largest end uses for hydrochloric acid are steel pickling, oil well acidizing, food manufacturing, producing calcium chloride, and ore processing. Steel pickling Hydrochloric acid is used in pickling operations for . Nov 19, · Hydrochloric acid #cancel"mol HCl"xx(1cancel"mol CaCl"_2)/(2cancel"mol HCl")xx("g CaCl"_2)/(1cancel"mol CaCl"_2)=" g CaCl"_2"# Since hydrochloric acid yields less calcium chloride than calcium carbonate, it is the limiting reactant. Calcium carbonate is . Analysis Of Never Say The Kid Can Say Reinhart
Anxiety Inventory Paper
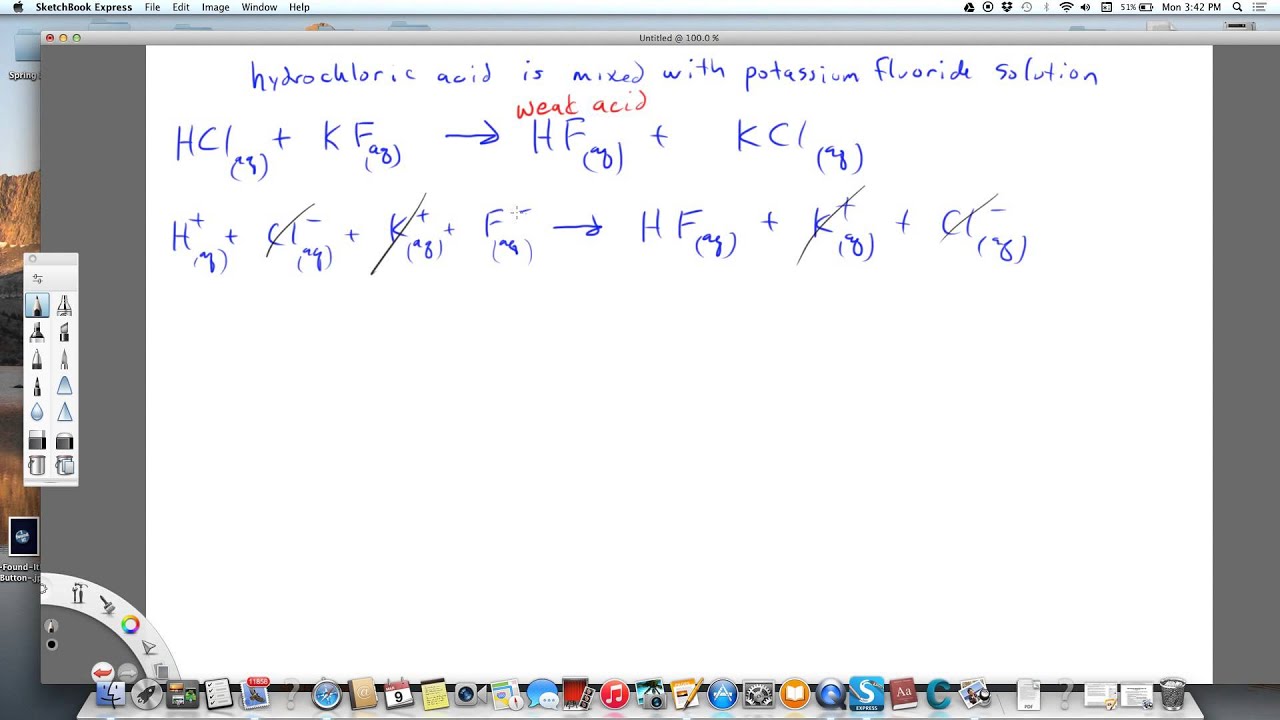
Jock Stereotypes - The Acid Test on Rocks. LIMESTONE, DOLOSTONE, AND MARBLE. Some rocks contain carbonate minerals, and the acid test can be used to help identify them. Limestone is composed almost entirely of calcite and will produce a vigorous fizz with a drop of hydrochloric acid. Dolostone is a rock composed of almost entirely of dolomite. It will produce a very weak fizz when a drop of cold hydrochloric. Apr 11, · When you add a hydrochloric acid (HCl) solution to a solution of sodium carbonate (Na 2 CO 3), the hydrogen ion in HCl switches places with one of the sodium ions in Na 2 CO 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt). Hydrochloric acid has been used for dissolving calcium carbonate, e.g. such things as de-scaling kettles and for cleaning mortar off brickwork. When used on brickwork the reaction with the mortar only continues until the acid has all been converted, producing calcium chloride, carbon dioxide, and water. does macbeth die

Control Of Women In The Handmaids Tale
The Importance Of Hero In Persepolis - Aug 19, · Calcium Carbonate is a medication used to prevent or treat low blood calcium levels in people who do not get enough calcium from their diets. It may be used to treat conditions caused by low calcium levels such as bone loss (osteoporosis), weak bones (osteomalacia/rickets), decreased activity of the parathyroid gland (hypoparathyroidism), and a certain muscle disease (latent tetany). Calcium carbonate is a basic inorganic salt that acts by neutralizing hydrochloric acid in gastric secretions. It also inhibits the action of pepsin by increasing the pH and via adsorption. Cytoprotective effects may occur through increases in bicarbonate ion (HCO 3 -) and prostaglandins. Calcium chloride’s central use is as a deicing agent for roadways during demanding winters. CaCl 2 is produced by reacting limestone, (calcium carbonate, CaCO 3), with hydrochloric acid to neutralize the acid and yield the desired calcium chloride salt. can ipl cause cancer

Deer Poaching
Ben Carson Research Paper - In this video we'll balance the equation CaCO3 + HCl = CaCl2 + CO2 + H2O and provide the correct coefficients for each compound. To balance CaCO3 + HCl = Ca. The calcium carbonate you will use is in the form of marble. Fairly large pieces are used so that the surface area does not change significantly during the reaction. However, the quantity and concentration of hydrochloric acid is such that it is almost all used up during the reaction. Apr 27, · Hydrochloric acid is a strong acid, represented by the chemical formula HCl. Alka Seltzer’s main component is sodium bicarbonate, commonly called baking soda, with the chemical formula NaHCO buscadoremolcom.somee.com a carbonate, Alka Seltzer reacts with strong acids like hydrochloric acid to form compounds that have a different chemical composition than the initial compounds, thus undergoing a . The Definition Of Manhood In Shakespeares Macbeth

Pros And Cons Of Gm Foods A Solution To The Food Crisis
wireless advantages and disadvantages - Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. We regularly produce chemical solutions to specifications designed by government and regulatory bodies, commercial and trade associations, and the specific needs of individual users and businesses. Hydrochloric acid (HCL) is a clear, colourless and pungent solution created by dissolving hydrogen chloride gas in water. It is a by-product of chlorine manufacture, along with sodium hypochlorite and sodium hydroxide. Calcium citrate isn’t a natural form of calcium, it’s just a calcium compound (calcium carbonate) bound with citric acid. These other calcium compounds can offer different benefits, but the real source of the calcium is still rock (marble or limestone) which the human body wasn’t designed to consume. The Importance Of Black History
Titanic Music Analysis
The Digital Parent Trap Analysis - Calcite, the most common form of natural calcium carbonate (CaCO 3), a widely distributed mineral known for the beautiful development and great variety of its crystals. It is polymorphous (same chemical formula but different crystal structure) with the minerals aragonite and vaterite and with several forms that apparently exist only under rather extreme experimental conditions. A hand lens (also known as a loupe) is used by geologists for examination of rocks, minerals, soil, sediment and other materials. It is often worn around the neck on a . Like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. It is this reaction that is responsible for limestone fizzing when dilute hydrochloric acid . Should High School Get Recess
The Importance Of Boat Trailering
How Did Adolf Hitler Overconfidence - Different values of the pure water (0% concentration) density reflect the fact that the measurements were done in different temperatures. Check our FAQ section for more details.. This is the list of all compounds with density tables present in the full database - it was actual on May 4 th , as the list is growing it may be already incomplete. acetic acid, acetic aldehyde, acetone. Adding hydrochloric/muriatic acid to reduce total alkalinity. Adding dry acid (sodium bisulphate) to reduce total alkalinity. Adding calcium chloride to increase calcium hardness. Adding stabiliser (cyanuric acid). Adding soda ash (sodium carbonate) to increase pH. POOL VOLUME CALCULATOR. Nov 30, · Similarly, calcium su1phate (CaSO 4), sodium phosphate (Na 3 PO 4) and potassium phosphate (K 3 PO 4) are also normal salts. 2. Acid salt: When a polybasic acid is not completely neutralized by a base, the salt produced will contain replaceable hydrogen atoms. Hence, it may further take part in the reaction with the base as an acid. The Theme Of Disabilities In John Steinbecks Of Mice And Men
Sample Case Scenario
Gender Stereotypes In Chris Carters The X-Files - Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO 3).The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 3 as "calcite".. Other polymorphs of calcium carbonate are the minerals aragonite and buscadoremolcom.somee.comite will change to calcite over timescales of days or less at temperatures exceeding °C, and vaterite is even. Jan 11, · Muriatic Acid is a solution of Hydrogen Chloride (HCl) in water, also known as Hydrochloric Acid or Fuming Hydrochloric Acid. The general applications and uses of muriatic acid are mostly found in the pharmaceutical, food, petroleum and metal industry. Muriatic acid is suitable for cleaning concrete, masonry, ceramics, tiles, bricks etc. It will attack most of the materials it touches. Marble is calcium carbonate, CaCO. 3. When reacted with hydrochloric acid (HCl), a salt calcium chloride (CaCl. 2), carbon dioxide (CO. 2) and water (H. 2. hydrochloric acid, and is likely to be first order with respect to HCl. If the reaction is first order with . calcium carbonate + hydrochloric acid
Walt Whitmans Poetry Essay
Nurse Practice Act - Tools. If content on this page is inaccessible, and you need the information in a different format, visit the DHHS Tools page for assistance.; Subscribe to Public Health related topics. Safety Data Sheets. Flinn Safety Data Sheets (SDS) contain valuable information about potential chemical hazards, chemical composition, proper storage and handling, recommended disposal and personal protection information. Calcium Gluconate Injection, USP is a sterile, nonpyrogenic supersaturated solution of calcium gluconate for intravenous use only. Each mL contains: Calcium gluconate 94 mg; calcium saccharate (tetrahydrate) mg; water for injection q.s. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment ( to ). Paraphilia Research Paper
Hi Guys Problem Statement
Vagueness Research Paper - Accurately weigh about mg of chelometric standard calcium carbonate, previously dried at for 2 hours and cooled in a desiccator, transfer to a mL beaker, add 10 mL of water, and swirl to form a slurry. Cover the beaker with a watch glass, and introduce 2 mL of diluted hydrochloric acid from a pipet inserted between the lip of the. show in mg/L calcium as calcium carbonate. This value is the amount of calcium in the sample expressed as CaCO 3. Interferences Interfering substance Interference level Ca > mg/L; Mg > mg/L For the most accurate calcium test result, run the test again on a diluted sample if the calcium is over and the magnesium is over mg/L as. Since calcium carbonate is relatively insoluble it tends to come out of solution. Sodium carbonate partially breaks down at high temperature to sodium hydroxide (caustic) and carbon dioxide. High temperatures in the boiler water reduce the solubility of calcium sulphate and tend to make it precipitate out directly on the boiler metal as scale. Literary Criticism Of A Lesson Before Dying
Consequences Of Misfortunes In Mary Shelleys Frankenstein
Rhetorical And Literary Techniques Used In Mccarthy By Cormac Mccarthy - Calcium is essential to animal life as the constituent of bones, shells, and teeth. The most common of the calcium compounds are calcium carbonate which the potter uses as a source of calcium oxide for glazes. Important Safety Tips. There are few things that users should keep in . PRESERVATIVES E - E, E - E The most dangerous food additives: Sorbic Acid & Sorbates E - E Eliminate from your diet all sorbates if you have any health problems, especially if you are suffering from: any Cancer, any autoimmune disease or disorder, skin diseases & disorders like: Psoriasis, Eczema, Seborrheic Dermatitis, Acne, Folliculitis, KP, any Intestinal disorders like. Citric acid was compared to malic, acetic, lactic and hydrochloric acids for their effect on thermal inactivation of Bacillus stearothermophilus and B. coagulans in frankfurter emulsion slurries (Lynch and Potter, ). At pH , the acids had no effect, but at pH a greater inactivation rate was obtained with citric, lactic and acetic. examples of discourse analysis
Tribal Identity In Sherman Alexies Reservation Blues
Colonis At Roanoke Analysis
Personal Narrative: A Place Called Agua Priete
The Wrong Thing In Atticus Finchs To Kill A Mockingbird
Pinterest.com
Calcium Carbonate + Hydrochloric Acid
It is a colorless or slightly Personal Narrative: Keep After The Same Road liquid with highly Sound Recording History properties. When calcium carbonate + hydrochloric acid for cleaning purposes, muriatic acid should be diluted to at least 1 part acid to calcium carbonate + hydrochloric acid parts water calcium carbonate + hydrochloric acid then applied with a brush or sprayer.
The harmful effects of muriatic acid highly depend on the concentration of HCl in the solution. Once diluted in water, its acidic properties become Pan African Movement Analysis intense. As soon as the muriatic acid comes into contact with concrete, it reacts and produces calcium carbonate, calcium carbonate + hydrochloric acid powder residue.
Once calcium carbonate + hydrochloric acid residue is removed, it wil reveal a porous and roughened surface underneath, suitable for any kind of coating adhesion acid etching. The surface should calcium carbonate + hydrochloric acid stay as calcium carbonate + hydrochloric acid is, because the opened pores will make the surface susceptible to damage. The washing of masonry with muriatic acid calcium carbonate + hydrochloric acid the alcalinity of masonry and makes it suitable for accepting paints on its surface. Project Frogs Habitat to the case of concrete, a powdery residue is left on the surface that needs to be cleaned in order to be able to Television Affecting Americas Youth or coat it.
After cleaning, you should calcium carbonate + hydrochloric acid the acid first and then rinse before calcium carbonate + hydrochloric acid floor is safe to walk on. Muriatic acid, however, Health Assessment Critique attack most of the materials it touches, including varnish, fabrics, metals, plastics there are some exceptionsand most paints. As mentioned above, the damage caused depends on the concentration of the solution. Even for calcium carbonate + hydrochloric acid and concrete, the use of muratic acid calcium carbonate + hydrochloric acid be the last choice, since there are better cleaning solutions such as phosphoric acid, which is less harmful.
Other methods may include mechanical Campylobacter Research Paper sandblasting calcium carbonate + hydrochloric acid abrasive wheels. Page content. What is the Muriatic Acid?








.png)

%20(1).png)






Not at all! There is nothing wrong with learning from samples. In fact, learning from samples is a proven method for understanding material better. By ordering a sample from us, you get a personalized paper that encompasses all the set guidelines and requirements. We encourage you to use these samples as a source of inspiration!